Electrochemical studies of decamethylferrocene in supercritical carbon dioxide mixtures
Abstract
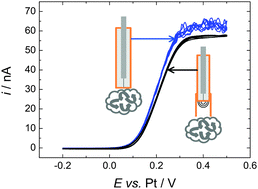
Detailed analysis of the voltammetry of decamethylferrocene at micro and macrodisc electrodes has been carried out in scCO2/MeCN (15 wt%), 20 mM [NBun4][BF4] and 309 K and 17.5 MPa. A passivating film needs to be removed from platinum electrodes before stable, reproducible voltammetry can be obtained. At low concentrations (0.22 mM) reversible 1e− behaviour is observed. Significant effects from natural convection are also present and it is demonstrated that fitting a baffle to the electrode dampens this effect. Limiting currents at microdisc electrodes at concentrations ranging from 0.22 to 11 mM and radii of 10 to 25 μm all obey the microdisc equation. The diffusion coefficient is calculated to be 4.06 × 10−5 cm2 s−1 in scCO2/MeCN (15 wt%) with 20 mM [NBun4][BF4] and 309 K at 17.5 MPa. The solubility of decamethylferrocene is in excess of 11 mM for these conditions.

 Please wait while we load your content...
Please wait while we load your content...