Graphene magnetism induced by covalent adsorption of aromatic radicals
Abstract
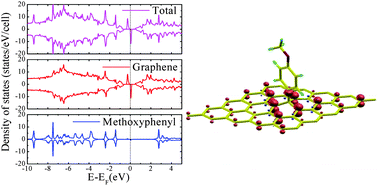
We report a computational study of adsorption of aromatic radicals onto graphene, with the aim of understanding the effect of covalent molecular functionalization on the magnetic and structural properties of graphene. Our results show that the adsorption of an aromatic radical like phenyl also functionalized with donor or acceptor groups generates a band gap and two spin-dependent midgap states, one located above and the other below the Fermi energy of pristine graphene, which cause a net magnetic moment. Due to the interaction between the radical and graphene, we find that the carbon atom on the adsorption site is lifted out of the graphene plane, and its pz orbital is removed from the π band system, leaving the electrons in the other sublattice unpaired, which results in nonzero magnetism. But the band gap of the full system is insensitive to the different attached species and the midgap states are independent of the alignment of the molecular orbitals, so that the magnetic moment is the same for the various radicals studied. The net result of the radical adsorption is to have almost the same aromatic species as those in the gas phase but anchored on a surface.

 Please wait while we load your content...
Please wait while we load your content...