Interaction of magnetic nanoparticles with phospholipid films adsorbed at a liquid/liquid interface
Abstract
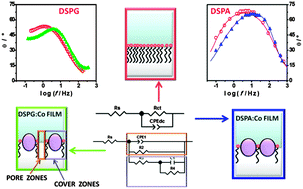
The interaction of Co hexagonal magnetic nanoparticles (MNPs) with distearoyl phosphatidyl glycerol (DSPG) and distearoyl phosphatidic acid (DSPA) films adsorbed at a water/1,2-dichloroethane interface is studied employing cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), capacity curves and interfacial pressure–area isotherms. DSPA and DSPG adsorb at the interface forming homogenous films and producing a blocking effect on the transfer process of tetraethyl ammonium (TEA+), used as a probe cation. In the presence of Co NPs this effect is reversed and the reversible transfer process for TEA+ is reestablished, to a greater or lesser extent depending on the structuration of the film. Co–DSPA hybrid films have a homogeneous structure while Co-DSPG films present different domains. Moreover, the presence of Co on DSPA film modifies the partition coefficient of the organic electrolyte into the hydrocarbon layer.

 Please wait while we load your content...
Please wait while we load your content...