Deactivation mechanism of a novel AIE-active naphthalimide derivative in more polar solutions
Abstract
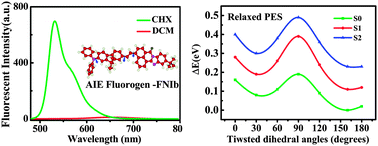
Aggregation-induced emission (AIE) compounds with intramolecular charge transfer (ICT) character have attracted considerable interest from experimental and theoretical researchers due to their potential in optoelectronic and sensory applications. However, their deactivation mechanism in solutions has been disputed, limiting their further applications. Therefore, in this work, we combined experimental observations with density functional theory (DFT) and time-dependent DFT (TD-DFT) methods to unveil the deactivation mechanism of a new AIE-active naphthalimide derivative (FNIb) in the polar solution, which was synthesized by us lately. An in-depth investigation on the effects of solvent polarity on its geometrical and electronic structures, and absorption and emission spectra indicates that FNIb would prefer a conformational planarization mechanism to the popular twisted intramolecular charge transfer mechanism (TICT) in the deactivation process. Our findings would add more insights into previous studies to rationalize the controversy on the deactivation mechanism of the AIE ICT-featured compounds in solutions.

 Please wait while we load your content...
Please wait while we load your content...