SO2 – yet another two-faced ligand†
Abstract
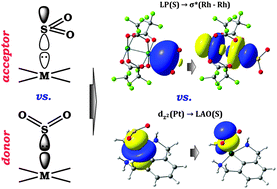
Experimentally known adducts of SO2 with transition metal complexes have distinct geometries. In the present paper, we demonstrate by a bonding analysis that this is a direct consequence of sulfur dioxide acting as an acceptor in one set, square-planar complexes of d8 and linear two-coordinated complexes of d10 transition metals, and as a donor with other compounds, well-known paddle-wheel [Rh2(O2CCF3)4] and square-pyramidal [M(CO)5] (M = Cr, W) complexes. Bonding energy computations were augmented by the natural bond orbital (NBO) analysis and energy decomposition analysis (EDA). When the SO2 molecule acts as an acceptor, bonding in the bent coordination mode to the axial position of the d8 or the d10 metal center, the dominant contributor to the bonding is LAO(S) (Lewis Acidic Orbital, mainly composed of the px-orbital of the S atom) as an acceptor, while a dz2 orbital centered on the metal is the corresponding donor. In contrast, the distinct collinear (or linear) coordination of the SO2 bound at the axial position of [Rh2(O2CCF3)4] and/or [M(CO)5] is associated with a dominant donation from a lone pair localized on the sulfur atom, σ*(Rh–Rh) and/or empty LAO(M) (mainly composed of the dz2 orbital of the metal), respectively, acting as an acceptor orbital. The donor/acceptor capabilities of the SO2 molecule were also checked in adducts with organic Lewis acids (BH3, B(CF3)3) and Lewis bases (NH3, N(CH3)3, N-heterocyclic carbene).

 Please wait while we load your content...
Please wait while we load your content...