Kinetics of stabilised Criegee intermediates derived from alkene ozonolysis: reactions with SO2, H2O and decomposition under boundary layer conditions
Abstract
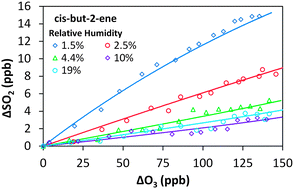
The removal of SO2 in the presence of alkene–ozone systems has been studied for ethene, cis-but-2-ene, trans-but-2-ene and 2,3-dimethyl-but-2-ene, as a function of humidity, under atmospheric boundary layer conditions. The SO2 removal displays a clear dependence on relative humidity for all four alkene–ozone systems confirming a significant reaction for stabilised Criegee intermediates (SCI) with H2O. The observed SO2 removal kinetics are consistent with relative rate constants, k(SCI + H2O)/k(SCI + SO2), of 3.3 (±1.1) × 10−5 for CH2OO, 26 (±10) × 10−5 for CH3CHOO derived from cis-but-2-ene, 33 (±10) × 10−5 for CH3CHOO derived from trans-but-2-ene, and 8.7 (±2.5) × 10−5 for (CH3)2COO derived from 2,3-dimethyl-but-2-ene. The relative rate constants for k(SCI decomposition)/k(SCI + SO2) are −2.3 (±3.5) × 1011 cm−3 for CH2OO, 13 (±43) × 1011 cm−3 for CH3CHOO derived from cis-but-2-ene, −14 (±31) × 1011 cm−3 for CH3CHOO derived from trans-but-2-ene and 63 (±14) × 1011 cm−3 for (CH3)2COO. Uncertainties are ±2σ and represent combined systematic and precision components. These values are derived following the approximation that a single SCI is present for each system; a more comprehensive interpretation, explicitly considering the differing reactivity for syn- and anti-SCI conformers, is also presented. This yields values of 3.5 (±3.1) × 10−4 for k(SCI + H2O)/k(SCI + SO2) of anti-CH3CHOO and 1.2 (±1.1) × 1013 for k(SCI decomposition)/k(SCI + SO2) of syn-CH3CHOO. The reaction of the water dimer with CH2OO is also considered, with a derived value for k(CH2OO + (H2O)2)/k(CH2OO + SO2) of 1.4 (±1.8) × 10−2. The observed SO2 removal rate constants, which technically represent upper limits, are consistent with decomposition being a significant, structure dependent, sink in the atmosphere for syn-SCI.

 Please wait while we load your content...
Please wait while we load your content...