Ligand(s)-to-metal charge transfer as a factor controlling the equilibrium constants of late first-row transition metal complexes: revealing the Irving–Williams thermodynamical series
Abstract
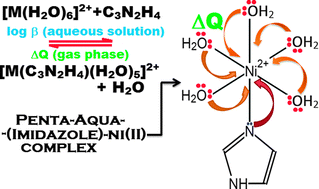
A unified relationship between the experimental formation constants and the ligand(s)-to-metal charge transfer values of versatile ligand complexes of late transition series first-row bivalent metal ions is uncovered. The latter property not only explicates the Irving–Williams series but also rationalizes quantitatively Pearson’s concept of hard and soft acids and bases by correlating the gas-phase to aqueous solution-phase chemistry in a broad sense.

 Please wait while we load your content...
Please wait while we load your content...