Construction of a series of lanthanide metal–organic frameworks: synthesis, structure, luminescence and white light emission†
Abstract
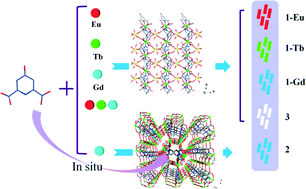
A series of new lanthanide metal–organic frameworks (Ln-MOFs) [Ln2(L)3(H2O)4]·2H2O (1) (Ln = Eu, Tb, Gd), and [Gd2(H2O)4(L)(C2O4)2]·3H2O (2) were synthesized based on 4-hydroxypyridine-2,6-dicarboxylic acid (H2L) under hydrothermal conditions. 1 exhibits isomorphous 2D wave-like networks constructed by paddle-wheel [Ln(L)3] units and LnO4(H2O)4 polyhedra. 2 shows a (4,4)-connected 3D framework with a Schlafli symbol of 66. Oxalic acid in the resulting framework of 2 is formed in situ under hydrothermal conditions. Luminescence studies indicate that 1-Eu and 1-Tb show characteristic red and green emissions of the corresponding Ln3+ ions, respectively, while 1-Gd and 2 exhibit blue emission arising from the H2L ligand. Then, by adjusting the co-doping ratio of three different Ln3+ ions into the same framework as that of 1, a novel doped Ln-MOF, [(Eu0.0073Tb0.0007Gd0.992)2(H2O)4(L)3]·2H2O (3), is successfully designed and synthesized, which shows white light emission upon excitation at 340 nm and its emission can also be switched between different colors. In addition, 1-Gd shows weak ferromagnetic interactions.

 Please wait while we load your content...
Please wait while we load your content...