Supramolecular interactions in boron hydrides: how non-classical bonding directs their crystal architecture†‡
Abstract
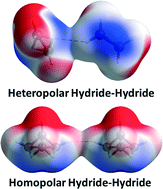
Boron hydrides typically contain both bridging B–H–B and terminal B–H bonds, which behave respectively as local Lewis acidic and basic sites. Their condensed phases are then stabilised through dihydrogen bonds between these hydride moieties. Here we explore the complex interplay of these H⋯H interactions for simple boranes, and we discuss the influence of 3c,2e B–H–B and B–B–B bonding on their extended structures. This analysis has also revealed a novel form of heteropolar dihydrogen bonding, involving an acidic B–H–B bridging and a basic terminal B–H moiety.

 Please wait while we load your content...
Please wait while we load your content...