Regulating silver morphology via electrochemical reaction
Abstract
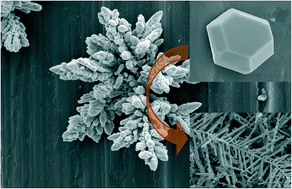
Controllable synthesis of materials with designed structures is a long-term dream of scientists and engineers. The challenges faced by dreamers are the complexity of material formation and the diversity of material structures. One way to discover this complexity is to simplify the reaction system. Electrochemical reduction is one of the simple reaction systems in which electrons are used as the reductant to avoid the complexity of chemical effects. We design a two-cell electrochemical reactor. The cathode and the anode are placed in different cells connected by a salt bridge. Electrons are introduced into the cell containing silver ions. With this approach, silver particles are synthesized on the cathode. The reduction rate of silver ions is regulated by varying the current density over two orders of magnitude. At various current densities, silver particles with different morphologies are synthesized. The dependence of silver morphology on current density is ascribed to the influence of current density on the nucleation and growth of silver particles, which is confirmed by subsequent experiments. The results in this paper show that the electron is a simple and green reductant and the current density is an effective tool for regulating reaction rates to shape materials.

 Please wait while we load your content...
Please wait while we load your content...