Mesoxalate as Cu(ii)–Ln(iii) linker in the construction of MOFs in DMSO/water medium†
Abstract
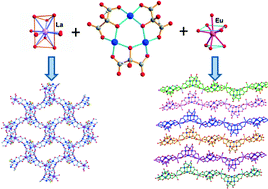
Five new heterometallic 3d–4f metal–organic frameworks with the general formula [Ln(H2O)zCu3(Hmesox)3DMSO]·xH2O·yDMSO (Ln(III) = La, Ce, Pr, Nd and Eu; H4mesox = mesoxalic or dihydroxymalonic acid; and DMSO = dimethylsulfoxide) have been prepared by crystal growth in agarose gel in a mixed solvent (DMSO/water) medium. LaCu3, CeCu3, PrCu3 and NdCu3 yield prismatic well-formed chiral crystals, whereas EuCu3 produces laminar and badly-shaped crystals. The structural analysis shows cubic neutral chiral (10,3)-a networks for the former four compounds. On the other hand, EuCu3 yields neutral chains with lower connectivity, crystallizing in a monoclinic space group. The magnetic susceptibility study reveals antiferromagnetic coupling among the copper(II) ions through the mesoxalate-alkoxo exchange pathway. Theoretical DFT studies agree with the magnetic susceptibility investigations and show a correlation between the intensity of the magnetic coupling and the bridging Cu–O–Cu angles.

 Please wait while we load your content...
Please wait while we load your content...