Controlled synthesis of mesocrystal magnesium oxide parallelogram and its catalytic performance
Abstract
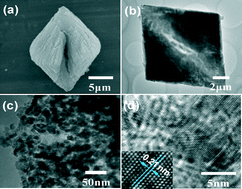
Mesocrystal magnesium oxide (MgO) parallelogram with lengths of diagonal line in the range of 7–9 μm has been synthesized through a facile precipitation method by using phosphate species as the morphology regulator and Mg(NO3)2 and Na2C2O4 as the inorganic sources. Scanning electronic microscope examination revealed that there is a canyon in the middle of the diagonal line of the as-synthesized products. The canyon resulted from the self-assembly of layer-like structures with a thickness of around 100–150 nm on both sides. To investigate the effect of reaction conditions on the morphologies and components of the resulting products, the types and addition amount of phosphate species and the reaction temperature were studied in detail. The results demonstrated that the introduction of trace amounts of phosphate species in the reaction system played a crucial role in determining the morphologies of the obtained products with variation of reaction temperatures, whereas their components have little been influenced after analyses by Fourier-transform infrared spectroscopy and X-ray diffraction. When as-synthesized mesocrystal MgO was employed as a catalyst in the Meerwein–Ponndorf–Verley (MPV) reaction between benzaldehyde and ethanol, it demonstrated superior performance to MgO particles without the presence of phosphate species.

 Please wait while we load your content...
Please wait while we load your content...