Effect of π–π stacking interactions on the emission properties of cadmium metal–organic frameworks based on 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene†
Abstract
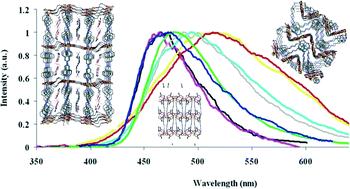
Five multidimensional cadmium metal–organic frameworks based on the luminescent 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene linker and flexible dicarboxylate ligands have been synthesized by conventional routes. These MOFs show fascinating structures and display, in the solid state and at room temperature, intense and hypsochromic photoluminescence properties when packed as a 3D network and bathochromic photoluminescence properties when arranged as 2D networks, as compared to the emission properties of the free luminescent 4-bpdb ligand. DFT calculations have revealed the establishment of destabilizing π–π stacking interactions between pyridyl rings of neighbouring 4-bpdb aromatic linkers on the 3D networks synthesized, responsible for the unexpected hypsochromic emission. The absence of π–π stacking interactions in the 2D MOFs yields the expected bathochromic photoluminescence arising from metal coordination with the aromatic ligand.

 Please wait while we load your content...
Please wait while we load your content...