Polymorphism and solid-to-solid phase transitions of a simple organic molecule, 3-chloroisonicotinic acid†
Abstract
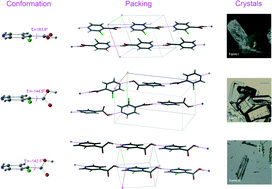
Three polymorphs (I, II, and III) have been discovered for 3-chloroisonicotinic acid. The torsion angle between the aromatic ring and the carboxylic acid in form I differs from that of forms II and III, which are similar. All three polymorphs form hydrogen-bonded chains based on the acid–pyridine heterosynthon. Despite the conformational similarity between forms II and III, the hydrogen-bonded chains in form II alternate in direction while those in form III all point in the same direction. Study of the phase behaviors of the three forms by differential scanning calorimetry, hot-stage microscopy, and thermogravimetric analysis revealed two solid-to-solid phase transitions from the metastable forms II and III to the most stable form I. Sublimation of 3-chloroisonicotinic acid also led to form I. A higher-temperature polymorph seemed to be possible but remained elusive. Lattice energy and hydrogen bonding strength calculations provided further insight into the stability of the polymorphs. A search of conformational space for the molecule suggested possibly additional polymorphs of this simple compound. The system may be valuable for further solid-state structure–property relationship studies.

 Please wait while we load your content...
Please wait while we load your content...