Relations between compression and thermal contraction in 1,2,4-trichlorobenzene and melting of trichlorobenzene isomers†
Abstract
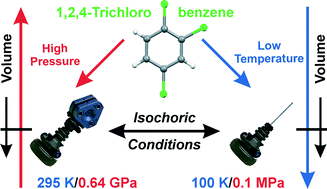
The compression and thermal expansion of crystalline 1,2,4-trichlorobenzene, C6H3Cl3, 124TCB, investigated under isobaric and isothermal conditions, are in reverse relation, as for most of crystals, however, the isochoric strain along direction c is clearly different from those along a and b. Single crystals of 124TCB have been in situ grown under isochoric and isobaric conditions, at 270 K/0.1 MPa and 295 K/0.16 GPa, and also at 100 K/0.1 MPa and 295 K/0.64 GPa, when the unit-cell volume is similar. All crystallizations yielded the same phase, of monoclinic space group P21/n, with two symmetry-independent molecules (Z′ = 2). The structure is governed by Cl⋯Cl and Cl⋯H interactions and the molecules are all nearly parallel to the [001] direction. Systematic differences of these specific contacts afford rationalizing the isochoric strain of the crystal. The phase diagram of 124TCB has been outlined.

 Please wait while we load your content...
Please wait while we load your content...