Structural diversities and magnetic properties of azide-containing coordination polymers based on flexible tetra-pyridinate ligands†
Abstract
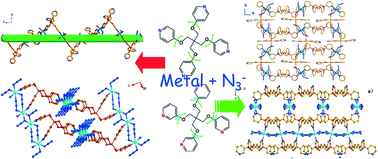
By utilizing two similar flexible quadritopic ligands, tetrakis(4-pyridyloxymethyl)methane (4-TPOM) and tetrakis(3-pyridyloxymethyl)methane (3-TPOM), four new azido-containing coordination polymers with formulas [Mn3(4-TPOM)3(N3)6(H2O)6]n (1), [Ni(4-TPOM)(N3)2]n (2), [Mn(3-TPOM)2(N3)2(H2O)3(CH3OH)4]n (3) and [Co(3-TPOM)(N3)2]n (4) have been synthesized and structurally characterized. Compound 1 shows a 1D helical chain composed of six-coordinated MnII geometries and two-connected tetra-pyridinate ligands. Compound 2 exhibits a three-dimensional framework based on 1D Ni(II) chains with alternating double end-to-end (EE) and double end-on (EO) azide bridges and tetrapyridyl ligands in a highly distorted conformation. Compound 3 shows a 2D layer composed of six-coordinated MnII geometries with mono-dentate azide ions and two types of two-connected tetra-pyridinate ligands. Compound 4 exhibits a three-dimensional framework based on 1D Co(II) chains with double end-to-end (EE) azide bridges and tetrapyridyl ligands in a cross-shaped conformation. Magnetic investigations on complexes 2 and 4 revealed antiferromagnetic interactions through the bridged azide ligands. In addition, the magnetic measurement reveals that 2 exhibits weak antiferromagnets, which might be caused by spin canting.

 Please wait while we load your content...
Please wait while we load your content...