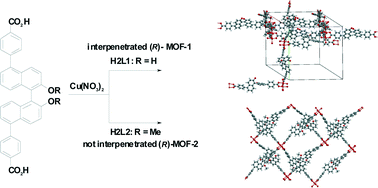
Interpenetrated and non-interpenetrated homochiral metal–organic frameworks based on (R)-2,2′-dihydroxy-1,1′-binaphthyl-5,5′-dibenzoic acid†
Abstract
A new example of interpenetration that is simply controlled by modifying hydroxyl function in homochiral MOFs is reported. Interpenetrated and non-interpenetrated chiral metal–organic frameworks (MOF-1 and MOF-2) were prepared by solvothermal reactions of [Cu(NO3)2]·3H2O in N,N-dimethylformamide (DMF)–H2O solution with (R)-2,2′-dihydroxy-1,1′-binaphthyl-5,5′-dibenzoic acid (H2L1) and (R)-2,2′-dimethoxy-1,1′-binaphthyl-5,5′-dibenzoic acid (H2L2), respectively. Interpenetrated chiral MOF-1 exhibited much higher catalytic performance in the Diels–Alder reaction of acrolein and 1,3-cyclohexadiene than non-interpenetrated chiral MOF-2.

 Please wait while we load your content...
Please wait while we load your content...