Construction of Zn(ii) and Cd(ii) metal–organic frameworks of diimidazole and dicarboxylate mixed ligands for the catalytic photodegradation of rhodamine B in water†
Abstract
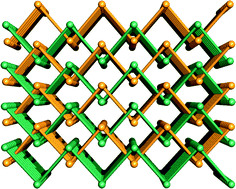
A set of four metal–organic frameworks, namely, [Zn(1,3-BDC)(bmimb)]n (1), [Zn(1,4-BDC)(bmimb)]n (2), [Cd(1,3-BDC)(bmimb)]n (3), and [Cd(1,4-BDC)(bmimb)]n (4), have been prepared under solvothermal conditions (1,3-BDC = 1,3-benzenedicarboxylate; 1,4-BDC = 1,4-benzenedicarboxylate; bmimb = 4,4′-bis(4-methyl-1-imidazolyl)biphenyl). The long bmimb ligand (N⋯N separation of ca. 14.1 Å) induces interpenetration of 1, 2 and 4 to increase both the framework stability and the density of effective catalytic metal centers. Compounds 1, 2 and 4 are interpenetrated 3D structures while 3 features a 2D structure. Compound 1 exhibits a parallel 2D → 3D polycatenation while 2 and 4 are isomorphous and feature 3D → 3D interpenetration. Compounds 1–4 fluoresce in the range of 350–372 nm in the solid state, whereas compounds 2 and 3 catalyze the almost complete photodegradation of rhodamine B (RhB) in water within ca. 4 h and can be recycled at least five times without loss in their crystallinity.

 Please wait while we load your content...
Please wait while we load your content...