Thermodynamics and preliminary pharmaceutical characterization of a melatonin–pimelic acid cocrystal prepared by a melt crystallization method†
Abstract
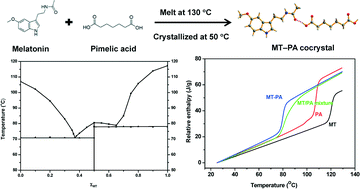
Pharmaceutical cocrystals have been extensively investigated as a promising approach to improve drugs' physicochemical properties. Cocrystals are usually prepared using solution-mediated methods and grinding methods. In this study, a cocrystal of pimelic acid with poorly soluble melatonin was produced using a melt crystallization method by controlling the crystallization within a specific temperature range. The cocrystal was characterized by infrared spectroscopy, powder and single crystal X-ray diffraction. The binary phase diagram and the formation enthalpy established by differential scanning calorimetry provided the rationale for this spontaneous cocrystallization system. The cocrystal exhibited higher apparent solubility, a faster dissolution rate and acceptable stability as compared to the original melatonin. The study has shown that the melt crystallization method can be considered as a competitive route for producing pharmaceutical cocrystals with improved properties and the thermodynamic investigation can provide in depth understanding and guidance on the melt crystallization of cocrystals.

 Please wait while we load your content...
Please wait while we load your content...