Enhanced gas-sorption properties of a high surface area, ultramicroporous magnesium formate†
Abstract
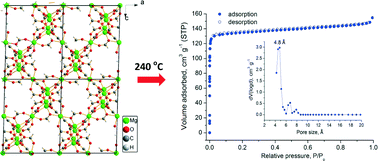
The direct solvothermal reaction of Mg(NO3)2·6H2O in dimethylformamide at 130 °C leads to the formation of highly crystalline α-[Mg3(O2CH)6] with an expanded unit cell. Successful pore activation was performed after heating the material at 240 °C under dynamic vacuum. In contrast to literature reports, the pores in the activated solid are accessible to nitrogen molecules and the corresponding BET surface area is 496 m2 g−1, very close to the geometric area calculated from the single crystal structure. A detailed gas-sorption study (N2, Ar, H2, CO2, CH4 and NH3) is reported. The material is ammonia stable showing a reversible uptake of 5.4 mmol g−1 at 298 K, while the corresponding CO2 uptake is 2.2 mmol g−1 with moderate CO2/CH4 (4.6) selectivity but high CO2/N2 (23) and CH4/N2 (5.2) selectivity. The latter is among the highest values reported to date in the entire family of porous materials. The H2 uptake at 77 K is 1.2 wt% which is the highest among the reported values for various magnesium formates.
- This article is part of the themed collection: Metal-Organic Frameworks and Hybrid Materials

 Please wait while we load your content...
Please wait while we load your content...