Flux-mediated crystal growth of metal oxides: synthetic tunability of particle morphologies, sizes, and surface features for photocatalysis research
Abstract
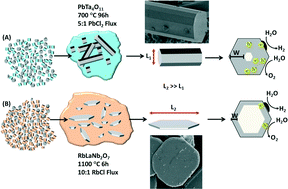
Molten-salt reactions can be used to prepare single-crystal metal-oxide particles with morphologies and sizes that can be varied from the nanoscale to the microscale, subsequently enabling a growing number of novel investigations into their photocatalytic activities. Crystal growth using flux-mediated methods facilitates finer synthetic manipulation over particle characteristics. The synthetic flexibility that flux synthesis affords for the growth of metal-oxides has led to the stabilization of phases with limited stability, the discovery of new compositions, and access to alternate crystal morphologies and sizes that exhibit significant changes in photocatalytic activities at their surfaces, such as for the reduction of water to hydrogen in aqueous solutions. This approach has significantly impacted the current understanding of the optical and photocatalytic properties of metal-oxides, such as the dependence of band gap energies on the structure and chemical composition (i.e., obtained from flux-mediated ion-exchange reactions). Thus, flux preparations of metal-oxide photocatalysts assist in the growth and optimization of their particles in order to understand and tune the photocatalytic reaction rates at their surfaces.

 Please wait while we load your content...
Please wait while we load your content...