Experimental and mechanistic analysis of the palladium-catalyzed oxidative C8-selective C–H homocoupling of quinoline N-oxides†
Abstract
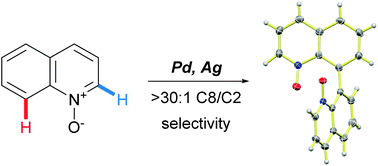
A novel site-selective palladium-catalyzed oxidative C8–H homocoupling reaction of quinoline N-oxides has been developed. The reaction affords substituted 8,8′-biquinolyl N,N′-dioxides that can be readily converted to a variety of functionalized 8,8′-biquinolyls. Mechanistic studies point to the crucial role of the oxidant and a non-innocent behavior of acetic acid as a solvent.

 Please wait while we load your content...
Please wait while we load your content...