Synthesis, molecular and electronic structure, and reactions of a Zn–Hg–Zn bonded complex†
Abstract
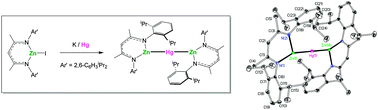
Reaction of (Ar′NacNac)ZnI with potassium/mercury amalgam gave the trimetallic compound {(Ar′NacNac)Zn}2Hg (1) containing a Zn–Hg–Zn unit and the first example of a bond between two different Group 12 metals; DFT and QTAIM analyses suggest that 1 is best described as two formally Zn(I) atoms with a Hg(0) atom positioned between them; reactions of 1 with stoichiometric I2, FpI or Fp2 gave addition products of the type (Ar′NacNac)ZnX (X = I, Fp) and Hg. Ar′NacNac = HC{C(Me)N(2,6-C6H3iPr2)}2; Fp = CpFe(CO)2.

 Please wait while we load your content...
Please wait while we load your content...