Supramolecular chemical shift reagents inducing conformational transitions: NMR analysis of carbohydrate homooligomer mixtures†
Abstract
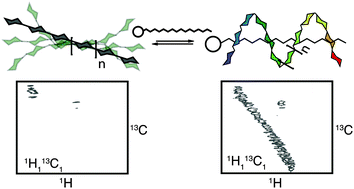
We introduce the concept of supramolecular chemical shift reagents as a tool to improve signal resolution for the NMR analysis of homooligomers. Non-covalent interactions with the shift reagent can constrain otherwise flexible analytes inducing a conformational transition that results in signal separation. Here we use this approach for the quantitative analysis of a complex homooligomeric glycan mixture.

 Please wait while we load your content...
Please wait while we load your content...