Self-selecting homochiral quadruple-stranded helicates and control of supramolecular chirality†
Abstract
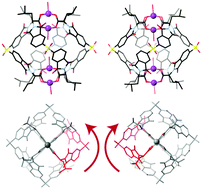
Enantiomeric M4L4 helical cages have been prepared whose supramolecular chirality is induced by the chemical chirality of the self-sorting amino acid-derived ligands that are used. Using scrambled diastereomeric ligands or achiral glycine-derived ligands yields analogous complexes yet ‘turns off’ the supramolecular chirality by producing centrosymmetric cages.


 Please wait while we load your content...
Please wait while we load your content...