A palladium-catalyzed tandem reaction of 2-(2-bromobenzylidene)cyclobutanone with 2-alkynylphenol†
Abstract
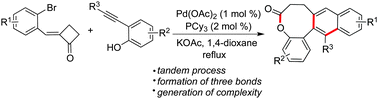
An efficient approach for the generation of benzo[b]naphtho[2,3-d]oxocin-6-ones through a palladium-catalyzed tandem reaction of 2-alkynylphenol with 2-(2-bromobenzylidene)cyclobutanone is described. This tandem process afforded the fused polycycles easily, with the formation of three bonds with high efficiency, starting from easily available materials. Good functional group tolerance as well as excellent selectivity was displayed.

 Please wait while we load your content...
Please wait while we load your content...