Diversity-oriented heterocyclic synthesis using divergent reactivity of N-substituted iso(thio)cyanates†
Abstract
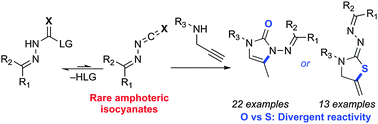
Carbon-substituted isocyanates and isothiocyanates are common building blocks in organic synthesis. In contrast, synthetic uses of N-substituted isocyanates and isothiocyanates are severely underdeveloped: few have been reported and their reactivity had not been compared. Herein, we compare the reactivity of blocked (masked) N-isocyanate and N-isothiocyanate precursors in cascade reactions. Divergent reactivity is observed with secondary propargylic and allylic systems, leading to new syntheses of imidazolones, thiazolidines, and a tool to form complex azomethine imines.

 Please wait while we load your content...
Please wait while we load your content...