Coupled molecular motions driven by light or chemical inputs: spiropyran to merocyanine isomerisation followed by pseudorotaxane formation†
Abstract
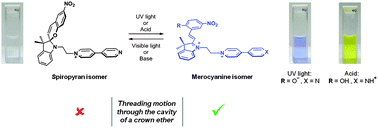
We have designed and prepared a new dual stimuli-responsive guest molecule containing a spiropyran fragment and a pyridinium moiety. Acid addition or UV-light irradiation induces guest transformation to a merocyanine isomer, promoting the threading motion through a 24-crown-8 macrocycle and the formation of a [2]pseudorotaxane complex.

 Please wait while we load your content...
Please wait while we load your content...