Phosphine-catalyzed regioselective Michael addition to allenoates†
Abstract
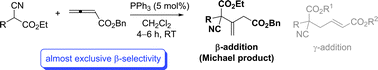
The first phosphine catalysed Michael addition of arylcyanoacetates to allenoates has been developed, and the β-selective products with a quaternary center were obtained in excellent yields. This unusual regioselectivity may open new opportunities to access interesting molecular structures.

 Please wait while we load your content...
Please wait while we load your content...