Intermolecular C–H activation with an Ir-METAMORPhos piano-stool complex – multiple reaction steps at a reactive ligand†
Abstract
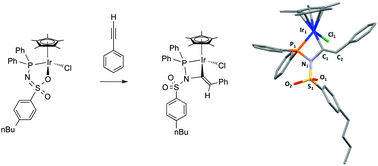
Substrate activation by means of a reactive ligand is a topic of much interest. Herein we describe a stoichiometric anti-Markovnikov C–N bond formation involving ligand reactivity in multiple steps along the reaction coordinate, including ligand assisted substrate (de)protonation and C–N bond formation, as illustrated by a combined experimental, spectroscopic and computational study. This affords a highly unusual four-membered iridacycle bearing an exo-cyclic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond.
C double bond.

 Please wait while we load your content...
Please wait while we load your content...