Total synthesis of (−)-depyranoversicolamide B†
Abstract
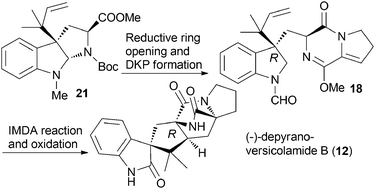
Starting from easily prepared (R)-C3-isoprenylated pyrroloindoline, the C3-isoprenylated indolyl diketopiperazine is prepared by an efficient reductive opening of the pyrrolo ring, and undergoes biomimetic Diels–Alder reaction to generate an anti-adduct as a sole stereoisomer. Oxidation of the indoline moiety to oxindole completes the synthesis of (−)-depyranoversicolamide B.

 Please wait while we load your content...
Please wait while we load your content...