Stereoselective synthesis of 2,3-disubstituted indoline, pyrrolidine and cyclic ether-fused 1,2-dihydroquinoline derivatives using alkyne iminium ion cyclization of vinylogous carbamates: switch of regioselectivity using an internal hydroxy group as a nucleophile†
Abstract
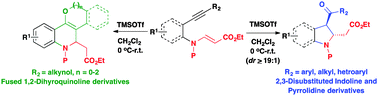
An intramolecular, alkyne iminium ion cyclization of vinylogous carbamates derived from o-alkynyl anilines and N-protected homopropargyl amines is developed for the stereoselective construction of trans-2,3-disubstituted indolines and pyrrolidine derivatives, respectively. The regioselectivity of the alkyne iminium ion cyclization could be switched using a hydroxy group as an internal nucleophile resulting in cyclic ether-fused 1,2-dihydroquinolines. The entire process of nitrogen heterocycle formation can also be carried out in a ‘one-pot’ manner starting from o-iodo aniline derivatives.

 Please wait while we load your content...
Please wait while we load your content...