Function and toxicity of amyloid beta and recent therapeutic interventions targeting amyloid beta in Alzheimer's disease
Abstract
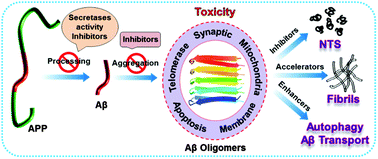
Amyloidogenesis has been implicated in a broad spectrum of diseases in which amyloid protein is invariably misfolded and deposited in cells and organs. Alzheimer's disease is one of the most devastating ailments among amyloidogenesis induced dementia. The amyloid beta (Aβ) peptide derived from amyloid precursor protein (APP) is misfolded and deposited as plaques in the brain, which are said to be the hallmark of Alzheimer's disease. In normal brains physiological concentration of the Aβ peptide has been indicated to be involved in modulating neurogenesis and synaptic plasticity. However, excess Aβ production, its aggregation and deposition deleteriously affect a large number of biologically important pathways leading to neuronal cell death. Targeting Aβ production, Aβ aggregation or its clearance from the brain has been an active area of research for preventing or curing AD. Our Feature Article intends to detail the aggregation mechanism, the physiological role of the Aβ peptide, elaborate its toxic effects, and outline the different classes of molecules designed in the last two years to inhibit amyloidogenic APP processing, Aβ oligomerization or fibrillogenesis and to modulate different pathways for active clearance of Aβ from the brain.
- This article is part of the themed collection: ChemComm 60th Anniversary Historic Papers from India

 Please wait while we load your content...
Please wait while we load your content...