The stereoselective formation of highly substituted CF3-dihydropyrans as versatile building blocks†
Abstract
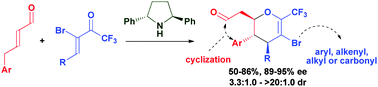
The highly enantioselective dienamine-mediated formation of 5-bromo-6-(trifluoromethyl)-3,4-dihydro-2H-pyrans from α,β-unsaturated aldehydes and α-bromo-(trifluoromethyl)-enones employing a C2-symmetric aminocatalyst is described. The products are demonstrated to be applicable in coupling reactions directly onto the ring, thereby granting access to a broad scope of highly substituted 6-(trifluoromethyl)-dihydropyran compounds.

 Please wait while we load your content...
Please wait while we load your content...