Coupling cyclizations with fragmentations for the preparation of heteroaromatics: quinolines from o-alkenyl arylisocyanides and boronic acids†
Abstract
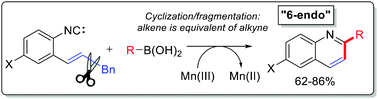
Stereoelectronic restrictions on homoallylic ring expansion in alkyne cascades can be overcome by using alkenes as synthetic equivalents of alkynes in reaction cascades that are terminated by C–C bond fragmentation. Implementation of this approach using Mn(III)-mediated reaction of o-alkenyl isocyanides and boronic acids leads to efficient synthesis of substituted quinolines.

 Please wait while we load your content...
Please wait while we load your content...