Direct photocatalytic fluorination of benzylic C–H bonds with N-fluorobenzenesulfonimide†
Abstract
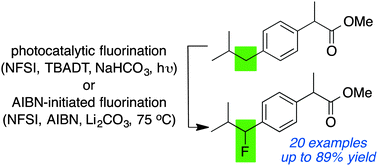
The late-stage fluorination of common synthetic building blocks and drug leads is an appealing reaction for medicinal chemistry. In particular, fluorination of benzylic C–H bonds provides a means to attenuate drug metabolism at this metabolically labile position. Here we report two complimentary strategies for the direct fluorination of benzylic C–H bonds using N-fluorobenzenesulfonimide and either a decatungstate photocatalyst or AIBN-initiation.

 Please wait while we load your content...
Please wait while we load your content...