Biophysical approaches for the study of interactions between molecular chaperones and protein aggregates
Abstract
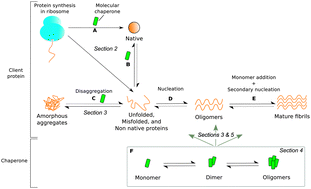
Molecular chaperones are key components of the arsenal of cellular defence mechanisms active against protein aggregation. In addition to their established role in assisting protein folding, increasing evidence indicates that molecular chaperones are able to protect against a range of potentially damaging aspects of protein behaviour, including misfolding and aggregation events that can result in the generation of aberrant protein assemblies whose formation is implicated in the onset and progression of neurodegenerative disorders such as Alzheimer's and Parkinson's diseases. The interactions between molecular chaperones and different amyloidogenic protein species are difficult to study owing to the inherent heterogeneity of the aggregation process as well as the dynamic nature of molecular chaperones under physiological conditions. As a consequence, understanding the detailed microscopic mechanisms underlying the nature and means of inhibition of aggregate formation remains challenging yet is a key objective for protein biophysics. In this review, we discuss recent results from biophysical studies on the interactions between molecular chaperones and protein aggregates. In particular, we focus on the insights gained from current experimental techniques into the dynamics of the oligomerisation process of molecular chaperones, and highlight the opportunities that future biophysical approaches have in advancing our understanding of the great variety of biological functions of this important class of proteins.

 Please wait while we load your content...
Please wait while we load your content...