Towards multifunctional MOFs – transforming a side reaction into a post-synthetic protection/deprotection method†
Abstract
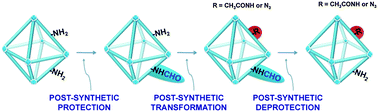
The contaminant commonly found in the important amino-substituted metal–organic framework UiO-66-NH2 has been shown to arise from partial formylation during the synthesis in DMF. Mild conditions have now been developed for both post-synthetic deformylation and near-complete formylation, offering a new post-synthetic protection–deprotection method for the synthesis of multifunctional MOFs.

 Please wait while we load your content...
Please wait while we load your content...