C–H functionalization of cyclic amines: redox-annulations with α,β-unsaturated carbonyl compounds†
Abstract
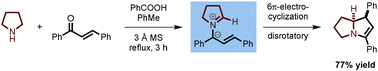
Cyclic amines such as pyrrolidine and 1,2,3,4-tetrahydroisoquinoline undergo redox-annulations with α,β-unsaturated aldehydes and ketones. Carboxylic acid promoted generation of a conjugated azomethine ylide is followed by 6π-electrocylization, and, in some cases, tautomerization. The resulting ring-fused pyrrolines are readily oxidized to the corresponding pyrroles or reduced to pyrrolidines.

 Please wait while we load your content...
Please wait while we load your content...