The dual role of borohydride depending on reaction temperature: synthesis of iridium and iridium oxide†
Abstract
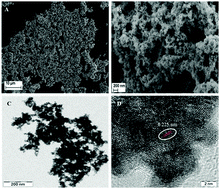
Temperature dependent reaction products are observed when borohydride is present in aqueous solutions containing Ir3+. At temperatures of 40 °C and above, metallic iridium is formed while under ambient conditions of 25 °C, borohydride results in an alkaline environment that helps in hydrolyzing the precursor to form IrO2. The Ir foams and IrO2 are subsequently used to study their catalytic properties.

 Please wait while we load your content...
Please wait while we load your content...