Synergistic Cu–amine catalysis for the enantioselective synthesis of chiral cyclohexenones†
Abstract
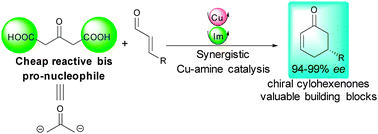
An unprecedented utilization of 1,3-acetonedicarboxylic acid as a 1,3-bis-pro-nucleophile and a reactive acetone surrogate in enantioselective catalysis has been reported. By synergistically activating the ketodiacid by copper catalysis and an α,β-unsaturated aldehyde by amine catalysis, an efficient domino di-decarboxylative Michael/aldol/dehydration sequence takes place leading to valuable chiral cyclohexenones in one single operation in 94 to 99% ee.

 Please wait while we load your content...
Please wait while we load your content...