Oxidant controlled regio- and stereodivergent azidohydroxylation of alkenes via I2 catalysis†
Abstract
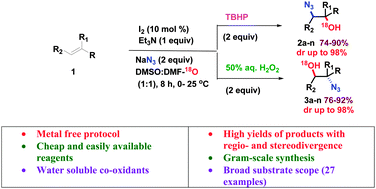
A novel, I2 catalyzed regio- and stereodivergent vicinal azidohydroxylation of alkenes leading to 1,2-azidoalcohols in high yields (up to 92%) and excellent dr (up to 98%) has been developed. This unprecedented transformation employs NaN3 and DMF as N- and O-nucleophiles respectively. The role of DMF as the O-source in the reaction has been unequivocally proven by 18O labelling studies.

 Please wait while we load your content...
Please wait while we load your content...