Palladium-catalyzed aerobic oxidative double allylic C–H oxygenation of alkenes: a novel and straightforward route to α,β-unsaturated esters†
Abstract
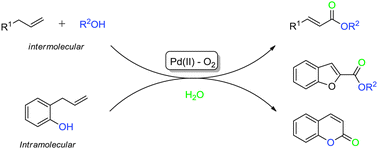
A mild tandem oxidative functionalization of allyl aromatic hydrocarbons was accomplished using the catalytic system of Pd(OAc)2/DMA under 1 atm O2. The green twofold C–O bond formation involving double allylic C–H oxygenation unlocks opportunities for markedly different synthetic strategies. Moreover, the reaction affords aryl α,β-unsaturated esters directly from readily available terminal olefins in moderate to good yields with excellent chemo- and stereoselectivities.

 Please wait while we load your content...
Please wait while we load your content...