The synthesis of carbonyl 2-amino-pyrimidines via tandem regioselective heterocyclization of 1,3-diynes with guanidine and selective oxidation†
Abstract
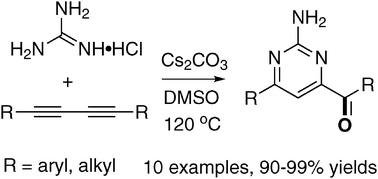
A highly efficient one-pot approach for the synthesis of carbonyl 2-amino-pyrimidines from 1,3-diynes and guanidine in the presence of Cs2CO3 and DMSO has been described. In these reactions, 1,3-diynes act as a precursor of buta-1,2,3-trienes and guanidine serves as the N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N source for the construction of pyrimidines. This methodology proves to be a tandem regioselective heterocyclization of 1,3-diynes with guanidine and selective oxidation with DMSO.
N source for the construction of pyrimidines. This methodology proves to be a tandem regioselective heterocyclization of 1,3-diynes with guanidine and selective oxidation with DMSO.

 Please wait while we load your content...
Please wait while we load your content...