DAST-promoted Beckmann rearrangement/intramolecular cyclization of acyclic ketoximes: access to 2-oxazolines, benzimidazoles and benzoxazoles†
Abstract
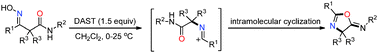
The first example of DAST-promoted Beckmann rearrangement/intramolecular cyclization of acyclic ketoximes is described. This unique protocol represents a direct and effective pathway to 2-oxazolines, benzimidazoles and benzoxazoles in moderate to good yields.

 Please wait while we load your content...
Please wait while we load your content...