Divergent synthesis of chiral heterocycles via sequencing of enantioselective three-component reactions and one-pot subsequent cyclization reactions†
Abstract
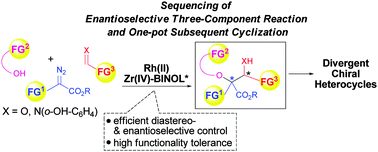
A highly efficient sequencing of catalytic asymmetric three-component reactions of alcohols, diazo compounds and aldimines/aldehydes with one-pot subsequent cyclization reactions was reported. The development of a robust and versatile Rh(II)/Zr(IV)–BINOL co-catalytic system not only gives high diastereo- and enantioselective controls of the three-component reaction, but also shows excellent functionality tolerances that allow a wide range of functionalities to be pre-installed in each component and readily undergo one-pot subsequent cyclization reactions, thus providing rapid and diversity-oriented synthesis (DOS) of different types of chiral nitrogen- and/or oxygen-containing polyfunctional heterocycles.

 Please wait while we load your content...
Please wait while we load your content...