Organocatalyzed oxidative N-annulation for diverse and polyfunctionalized pyridines†
Abstract
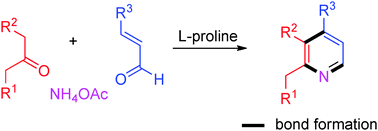
This paper describes a novel strategy for the synthesis of 2,3,4-trisubstituted pyridines via organocatalyzed three-component reactions. A variety of pyridine derivatives are synthesized from readily available ketones with α,β-unsaturated aldehydes and ammonium acetate under a mild organocatalyst. This protocol leads to rapid N-annulation through C–C and C–N bond formation in a single operation, thereby avoiding the preparation of essential functional groups, such as oximes, imines, or azides. The synthesized compounds are used for the evaluation of antibacterial activities and as fluorescence sensors for Cu2+ ions.

 Please wait while we load your content...
Please wait while we load your content...