Generation of cycloheptynes and cyclooctynes via a sulfoxide–magnesium exchange reaction of readily synthesized 2-sulfinylcycloalkenyl triflates†
Abstract
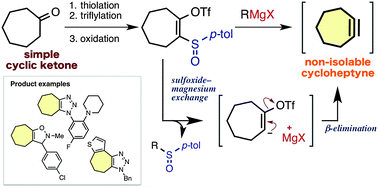
Cycloheptynes and cyclooctynes were efficiently generated via a sulfoxide–magnesium exchange reaction of readily synthesized 2-sulfinylcycloalkenyl triflates. Cycloadditions between various ynophiles and the cycloalkynes generated by this method proceeded efficiently, providing an easy method to prepare a wide range of heterocycles fused with seven- or eight-membered carbocycles.

 Please wait while we load your content...
Please wait while we load your content...