Stereocontrolled synthesis of vicinally functionalized piperidines by nucleophilic β-addition of alkyllithiums to α-aryl substituted piperidine enecarbamates†
Abstract
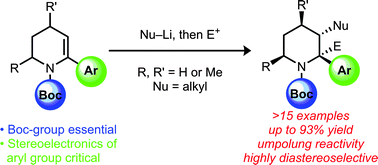
Substituted piperidines are emerging as important medicinally-active structural motifs. Here, we report highly stereoselective carbolithiation reactions of α-aryl piperidine enecarbamates that offer direct access to vicinally-substituted piperidine compounds. We have also demonstrated that the carbanion intermediates can be trapped with a carbon electrophile.

 Please wait while we load your content...
Please wait while we load your content...