1,4,8,11,15,18,22,25-Alkylsulfanyl phthalocyanines: effect of macrocycle distortion on spectroscopic and packing properties†
Abstract
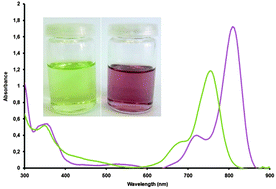
The effect of phthalocyanine macrocycle distortion on its spectroscopic and packing properties is studied, by comparing two phthalocyanines octa-non-peripherally substituted by alkanethiols of different bulkiness (n-hexyl and tert-butyl). Their X-ray structures evidence their core shape, respectively planar and strongly distorted, inducing a 55 nm shift of their maximum absorption wavelength. Comparison of frontier orbital energies revealed that this distortion decreases the conjugation potency of the benzo rings to the central pyrrolic rings. Also the tert-butyl derivative presents a MOF-like porous crystalline assembly with 22.2% void.

 Please wait while we load your content...
Please wait while we load your content...